It would appear (to the jaded and cynical) that there is an unspoken rule in mainstream optometry diagnosis / treatment. This rule is simple: Anything goes, as long as a patented / branded product is being sold in the treatment process.
And there would be a the second part of the secret rule: Your product has to exonerate any previous products used for treating the same symptom, implicitly or otherwise. Meaning, your product has to somehow be newer, better, and in no way suggesting that older products were doing something not exactly benign.
Let’s translate this to the myopia topic:
Do you want to market bi-focal contact lenses as myopia treatment?
Can you make that work? Let’s see if it fits the rules:
Branded product sale? Check.
Not casting a negative light on previous products? Yes, also check.
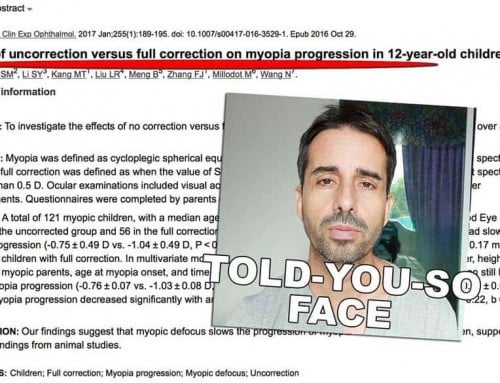
Visit scholar.google.com and search for myopia control studies, look at the pattern, and see how it all makes sense. Studies looking at whether less lens wear causes less myopia? Breaking the rule. Results? Doesn’t work. How about studies honoring the rules we just discussed?
Here’s one, topical and appropriate:
Here’s the full PDF of this particular study.
If you read this site regularly, you already know why bi-focals work to a limited extent, and why that method amounts to just a half measure. And yet, it’s a start. As long as myopia control is becoming an increasing part of the dialog, we are headed in the right direction.
Let’s look for more M.D.’s picking up the banner and talking about ways to #endmyopia.
Happy Sunday.
Cheers,
-Jake


![twins-bi-focal-myopia-study]](https://endmyopia.org/wp-content/uploads/2015/11/twins-bi-focal-myopia-study.jpg)